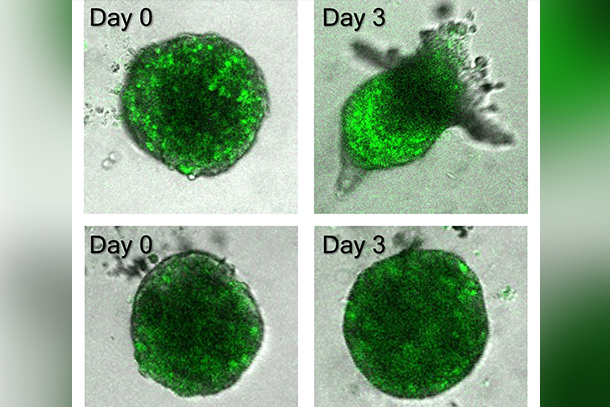
A Penn State-led research team found that a molecule called MALAT1 helps regulate the cells that lead cancer invasion. The top row shows bladder cancer cells with MALAT1 expression, while the bottom row shows bladder cancer cells with reduced MALAT1 expression. In the top row, the cells are beginning their migration away from the tumor, while the bottom row shows no such migration. Credit: Wong Lab/Penn State. All Rights Reserved.
Follow the leader: Researchers identify mechanism of cancer invasion
June 26, 2023
By Ashley WennersHerron
UNIVERSITY PARK, Pa. — A cancerous tumor is the accumulation of cells uncontrollably dividing, some of which can invade other parts of the body. The process is difficult to predict in detail, and eradicating the cells poses even greater difficulty. Now, a Penn State-led research team has revealed how the exodus initiates, shedding light on a potential therapeutic target to halt the invasion and providing a prognostic marker to help clinicians select the best treatment option.
They published their findings today (June 26) in the Proceedings of the National Academy of Sciences.
“Cancer cells don’t randomly detach from the primary tumor and disseminate everywhere — they often exhibit coordination and collaboration,” said corresponding author Pak Kin Wong, professor of biomedical engineering, of mechanical engineering and of surgery at Penn State. “A leader cell may emerge to coordinate the invasion, thereby enhancing the efficiency of cancer cell dissemination. In this study, we found a molecular marker for leader cells that enables us to predict the invasiveness of the tumor and how they invade.”

Pak Kin Wong, professor of biomedical engineering, of mechanical engineering and of surgery at Penn State, led the study. Credit: Kate Myers / Penn State. All Rights Reserved.
In cancer cells derived from human patients with muscle invasive bladder cancer, the researchers designed a nanobiosensor to track long noncoding RNA (lncRNA), which refers to extensive lengths of genetic material that do not encode genes but regulate how a cell expresses them as proteins.
“lncRNA are often referred to as the dark matter of the cell,” Wong said. “While many RNA are involved in protein expression, lncRNA do not encode proteins. Their functions and how they regulate cell processes are still poorly understood. We developed this sensor to study lncRNA and their potential contribution to cancer progression.”
The sensor enables the researchers to identify and track individual lncRNA molecules of interest as they typically behave and function in cells. In conventional analysis approaches, Wong said, researchers usually cannot study how they function in space and time because the cells are typically fixed or broken apart.
Using the sensor, the researchers monitored how much and where lncRNA was distributed in the cells during collective cancer invasion. They found that MALAT1, a gene associated with metastasis in lung, bladder and other cancers, was highly present in leader cells. Importantly, Wong said, MALAT1 expression increased when a cancer cell became a leader and decreased when the leader cell was no longer needed — such as when the invasion process ceased or when it was replaced by another leader cell.
“We also found that reducing the expression of MALAT1 in cells prevents the formation of leader cells and abolishes the invasion of cancer cells,” Wong said. “Overall, our single-cell analysis suggests that MALAT1 plays an essential role in regulating leader cells during collective cancer invasion.”
Wong said the team will continue to study the mechanistic underpinnings of MALAT1 in leader cells, with the goal of providing a prognostic tool to guide treatment.
“If we can comprehend the crucial characteristics and functions of leader cells, we might be able to help clinicians identify aggressive disease and predict the behavior,” Wong said. “We hope this study will lead to the development of novel prognostics and therapeutic approaches targeting bladder and other cancer. For example, if applied clinically, determining the presence of leader cells and aggressive disease could enhance a physician's understanding of an individual patient's prognosis and inform the most suitable treatment strategy.”
Collaborators include Ninghao Zhu, who earned his doctorate degree in biomedical engineering from Penn State in 2022; Mona Ahmed, a doctoral student in biomedical engineering at Penn State; Yanlin Li, a doctoral student in electrical and electronics engineering at Penn State; and Joseph C. Liao, Kathryn Simmons Stamey Professor in Stanford University School of Medicine’s Department of Urology.
The National Science Foundation and the National Institutes of Health supported this work.



