
Sri-Rajasekhar “Raj” Kothapalli, assistant professor of biomedical engineering at Penn State, holds a transparent, biocompatible ultrasound transducer chip, that when hooked to power, stimulates cells. The new ultrasound setup has implications for future cancer and stem cell research. Credit: Kelby Hochreither/Penn State.
Transparent ultrasound chip improves cell stimulation and imaging
Feb 24, 2022
By Mariah Chuprinski
UNIVERSITY PARK, Pa. — Ultrasound scans, best known for monitoring pregnancies or imaging organs, can also be used to stimulate cells and direct cell function. A team of Penn State researchers has developed an easier, more effective way to harness the technology for biomedical applications.
The team created a transparent, biocompatible ultrasound transducer chip that resembles a microscope glass slide and can be inserted into any optical microscope for easy viewing. Cells can be cultured and stimulated directly on top of the transducer chip and the cells’ resulting changes can be imaged with optical microscopy techniques.
Published in the Royal Society of Chemistry’s journal Lab on a Chip, the paper was selected as the cover article for the December 2021 issue. Future applications of the technology could impact stem cell, cancer and neuroscience research.
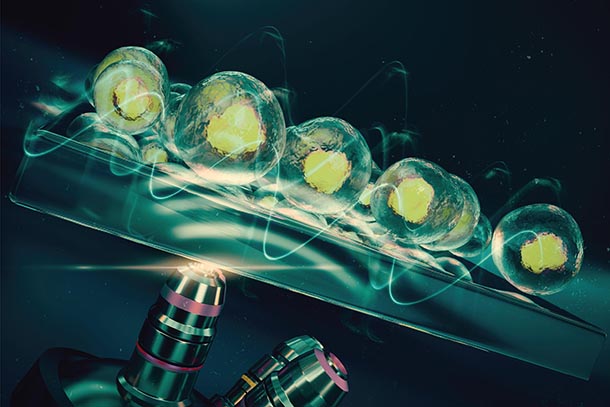
Kothapalli's paper was selected as the cover article for The Royal Society of Chemistry's journal Lab on a Chip. This image was used, which highlights the placement of the microscope, seen below the chip in the graphic, which allows for easy viewing and imaging. Credit: Sciencebrush.
“In the conventional ultrasound stimulation experiments, a cell culture dish is placed in a water bath, and a bulky ultrasound transducer directs the ultrasound waves to the cells through the water medium,” said Sri-Rajasekhar “Raj” Kothapalli, principal investigator and assistant professor of biomedical engineering at Penn State. “This was a complex setup that didn’t provide reproducible results: The results that one group saw another did not, even while using the same parameters, because there are several things that could affect the cells’ survival and stimulation while they are in water, as well as how we visualize them.”
Kothapalli and his collaborators miniaturized the ultrasound stimulation setup by creating a transparent transducer platform made of a piezoelectric lithium niobate material. Piezoelectric materials generate mechanical energy when electric voltage is applied. The chip’s biocompatible surface allows the cells to be cultured directly on the transducer and used for repeated stimulation experiments over several weeks.
When connected to a power supply, the transducer emits ultrasound waves, which pulse the cells and trigger ion influx and outflux.
To test the setup, Kothapalli and his team cultured bladder cancer cells on the chip. They then inserted fluorescent calcium indicators into the cells to allow researchers to clearly see dynamic changes in cell calcium signaling under the microscope during stimulation.
“Since the cells are directly sitting on the transparent transducer surface, we can confirm that all the cells are equally stimulated at the same time using a single ultrasound stimulus, unlike conventional approaches,” Kothapalli, a co-hire with the Penn State Cancer Institute, said. “And unlike earlier processes, we can get high resolution images of many cells at once in a single field of view, because we are able to see the cells from a close distance.”
Through the bladder cancer cell study, researchers established proof-of-concept for the new transducer setup. But they can extend these findings to use the transducer setup in potential future applications, according to Kothapalli, such as stem cell differentiation, mechanosensitive neuromodulation, drug delivery and the opening of the blood-brain barrier.
“This simple setup will be invaluable for researchers interested in modulating cells and tissues with an ultrasound,” said Pak Kin Wong, professor of biomedical engineering, mechanical engineering and surgery at Penn State and co-author on the paper. “It can be used to explore novel therapeutic ultrasound applications, such as focused ultrasound immunotherapy.”
The ultrasound stimulation chip is low-cost, easy to fabricate, compact and scalable in size, and disposable and reusable, according to Haoyang Chen, first author of the paper and doctoral student under Kothapalli in biomedical engineering.
“It is easy to grow cells on the chip using standard cell culturing methods,” Chen said. “The setup provides controllable stimulation parameters for a variety of experiments and can be imaged with all conventional optical microscopy techniques.”
In addition to Kothapalli, Wong and Chen, other contributors to the study were Peter Butler, Penn State professor of biomedical engineering and associate dean for education and graduate professional programs; biomedical engineering graduate students Ninghao Zhu, Mohamed Osman and Shubham Khandare; and biomedical engineering undergraduate students Ryan Biskowitz and Jinyun Liu.
The study was partially funded by the Penn State Cancer Institute, a Penn State multidisciplinary seed grant, and the National Science Foundation.




