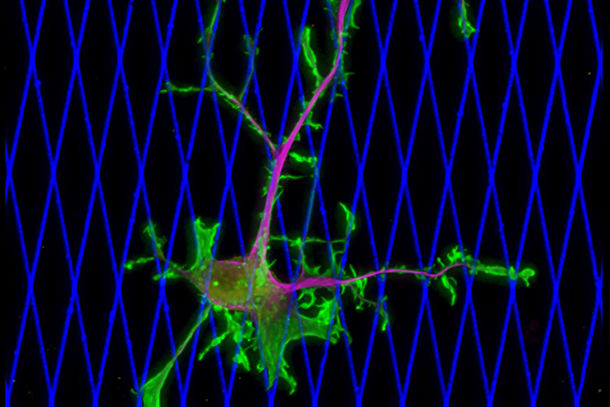
A human breast cancer cell, adenocarcinoma MDA-MB-231, demonstrates metastatic-like adhesion, spreading and migrating in a collagen matrix designed to mimic soft tissue. New research led by Penn State reveals for the first time the mechanics behind how breast cancer cells may invade healthy tissues. The discovery, showing that a motor protein called dynein powers the movement of cancer cells in soft tissue models, offers new clinical targets against metastasis and has the potential to fundamentally change how cancer is treated. Credit: Courtesy Erdem Tabdanov/Penn State
Mechanics of breast cancer metastasis discovered, offering target for treatment
October 27, 2023
By Adrienne Berard
Editor's note: This article originally appeared on Penn State News.
UNIVERSITY PARK, Pa. — The most lethal feature of any cancer is metastasis, the spread of cancer cells throughout the body. New research led by Penn State reveals for the first time the mechanics behind how breast cancer cells may invade healthy tissues. The discovery, showing that a motor protein called dynein powers the movement of cancer cells in soft tissue models, offers new clinical targets against metastasis and has the potential to fundamentally change how cancer is treated.
“This discovery marks a paradigm shift in many ways,” said Erdem Tabdanov, assistant professor of pharmacology at Penn State and a lead co-corresponding author on the study, recently published in the journal Advanced Science. “Until now, dynein has never been caught in the business of providing the mechanical force for cancer cell motility, which is their ability to move themselves. Now we can see that if you target dynein, you could effectively stop motility of those cells and, therefore, stop metastatic dissemination.”
The project began as a collaboration between Penn State’s Department of Chemical Engineering and Penn State’s College of Medicine, before growing into a multi-institution partnership with researchers at the University of Rochester Medical Center, Georgia Institute of Technology, Emory University, and the U.S. Food and Drug Administration.
The researchers used live microscopy to watch the migration of live breast cancer cells in two different systems modeled after the human body. The first system, a two-dimensional network of collagen fibers, revealed how cancer cells move through an extra cellular matrix that surrounds tumors and showed that dynein was key to the movement of cancer cells. The second system was a three-dimensional model developed by a team led by Amir Sheikhi, Dorothy Foehr Huck and J. Lloyd Huck Early Career Chair in Biomaterials and Regenerative Engineering and assistant professor of chemical engineering and biomedical engineering at Penn State.
Human breast cancer cells are seen migrating within a 3D model for healthy soft tissue, designed by Amir Sheikhi of Penn State. The microgels are invisible to avoid visual interference with the cells. The nuclei of the cells are green. Credit: Erdem Tabdanov/Penn State
“The second system was designed to mimic soft tissue using a network of microscopic hydrogel particles or microgels linked together in tumor-like shapes. Like in the two-dimensional model, the researchers found in the three-dimensional model that dynein was “indispensable” in the spread or metastasis of cancer cells.
“Using these three-dimensional models that partially mimic a tumor, we discovered that if we block the dynein, the cancer cells cannot effectively move and infiltrate solid tissues,” Sheikhi said. “In both models, we found that dynein is extremely important for cell locomotion, which suggests a whole new method for cancer management. Instead of killing the cancer cells with radiation or chemotherapy, we are showing how to paralyze them. This is great news because you don't really have to kill the cells, which is a harsh approach that targets both cancerous and healthy cells. Instead, you just have to stop the cancer cells from moving.”
Tabdanov explained that cell “paralysis” could prove to be an effective treatment strategy for cancer compared to chemotherapeutic treatments, because after surgical removal of the main tumor, it could prevent the cancer from spreading without damaging healthy tissues and cells.
“The trick with chemotherapy is to kill the cancer cells slightly faster than the rest of the body — it’s a race against time,” Tabdanov said. “Chemotherapy causes a lot of damage to the body’s normal, healthy tissues while it is busy killing the cancer. If we instead contained the cancer, stopped it in its tracks, we could keep the healthy parts of the body healthy.”
The researchers noted that any potential clinical treatment is still far off — as they have yet to run human or animal trials. Sheikhi has filed multiple patents related to his team’s platform and plans to use the technology to study a myriad of diseases, including other cancers.
“We are very excited about this collaboration with the Penn State College of Medicine, and our labs are working closely on other projects,” Sheikhi said. “I think these platforms could one day enable personalized medicine and personalized treatment for cancer and, hopefully, many other diseases.”
Other authors on the paper are Yerbol Tagay of Penn State College of Medicine; Sina Kheirabadi and Zaman Ataie of Penn State’s Department of Chemical Engineering; Rakesh Singh of the University of Rochester Medical Center; Denis Tsygankov of Georgia Institute of Technology and Emory University; and Olivia Prince, Ashley Nguyen, Alexander Zhovmer and Xuefei Ma of the U.S. Food and Drug Administration.
Startup funds from the Department of Pharmacology at Penn State College of Medicine, the Meghan Rose Bradley Foundation, the National Science Foundation, the National Institutes of Health, and the U.S. Food and Drug Administration supported this work.



