
A team of scientists at Penn State found that the thicker mucus is, the better bacteria are able to organize into swarms to infect it. The findings could have implications for treatments that reduce the ability of bacteria to spread. Credit: Andrea Piacquadio/Creative Commons
Bacteria's mucus maneuvers: Study reveals how snot facilitates infection
New study shows thicker mucus supercharges bacteria’s ability to self-organize into swarms to spread infection
December 5, 2023
By Adrienne Berard
Editor’s note: This article first appeared on Penn State News.
UNIVERSITY PARK, Pa. — Sniffles, snorts and blows of runny noses are the hallmarks of cold and flu season — and that increase in mucus is exactly what bacteria use to mount a coordinated attack on the immune system, according to a new study from researchers at Penn State. The team found that the thicker the mucus, the better the bacteria are able to swarm. The findings could have implications for treatments that reduce the ability of bacteria to spread.
The study, recently published in the journal PNAS Nexus, demonstrates how bacteria use mucus to enhance their ability to self-organize and possibly drive infection. The experiments, performed using synthetic pig stomach mucus, natural cow cervical mucus and a water-soluble polymer compound called polyvidone, revealed that bacteria coordinate movement better in thick mucus than in watery substances.
The findings provide insight into how bacteria colonize mucus and mucosal surfaces, researchers said. The findings also show how mucus enhances bacterial collective motion, or swarming, which may increase antibiotic resistance of bacterial colonies.
Real time movie of individual bacteria swimming in mucus broth. Credit: Courtesy Igor Aronson.
“To the best of our knowledge, our study is the first demonstration of bacteria collectively swimming in mucus,” said Igor Aronson, Huck Chair Professor of Biomedical Engineering, of Chemistry and of Mathematics at Penn State and corresponding author on the paper. “We have shown that mucus, unlike liquids of similar consistency, enhances the collective behavior.”
Mucus is essential for many biological functions, explained Aronson. It lines the surfaces of cells and tissues and protects against pathogens such as bacteria, fungi and viruses. But it is also the host material for bacteria-born infections, including sexually transmitted and gastric diseases. A better understanding of how bacteria swarm in mucus could pave the way for new strategies to combat infections and the growing problem of antibiotic resistance, according to Aronson.
“Our findings demonstrate how mucus consistency affects random motion of individual bacteria and influences their transition to coordinated, collective motion of large bacterial groups,” Aronson said. “There are studies demonstrating that collective motion or swarming of bacteria enhances the ability of bacterial colonies to fend off the effect of antibiotics. The onset of collective behavior studied in our work is directly related to swarming.”
Mucus is a notoriously challenging substance to study because it exhibits both liquid-like and solid-like properties, Aronson explained. Liquids are typically described by their level of viscosity, how thick or thin the liquid is, and solids are described by their elasticity, how much force it can take before breaking. Mucus, a viscoelastic fluid, behaves as both a liquid and solid.
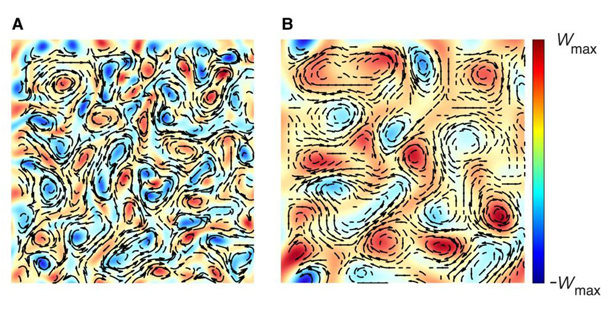
The left side shows computational modeling results of model bacteria undergoing polymer stress, or thicker mucus. The right side is not undergoing polymer stress. Credit: Provided by Igor Aronson
To better understand how mucus becomes infected, the team used microscopic imaging techniques to observe the collective motion of the concentrated bacteria Bacillus subtilis in synthetic pig stomach mucus and natural cow cervical mucus. They compared those results with observations of Bacillus subtilis moving in a water-soluble polymer polyvidone at a wide range of concentrations, from high to low levels of polyvidone. The researchers also compared their experimental results to a computational model for bacterial collective motion in viscoelastic fluids like mucus.
The team found that the consistency of mucus profoundly affects the collective behavior of bacteria. The results indicated that the thicker the mucus, the more likely the bacteria would exhibit collective movement, forming a coordinated swarm.
“We were able to show how the viscoelasticity in mucus enhances bacterial organization, which in turn leads to coherently moving bacterial groups that cause infection,” Aronson said. “Our results reveal that the levels of elasticity and viscosity in mucus are a main driver in how bacterial communities organize themselves, which can provide insight into how we can control and prevent bacterial invasion in mucus.”
Aronson explained that the team expects human mucus to exhibit similar physical properties, meaning their findings are also relevant for human health.
“The onset of the collective motion of bacteria and their interaction with mucus should be the same as in cow, pig or human mucus since these substances have similar mechanical properties,” Aronson said. “Our results have implications for human and animal health. We’re showing that mucus viscoelasticity can enhance large-scale collective motion of bacteria, which may accelerate how quickly bacteria penetrate mucus protective barrier and infect internal tissues.”
The other co-author on the paper is Wentian Liao, a doctoral candidate in biomedical engineering at Penn State. The National Science Foundation supported the work.



