Amir Sheikhi, assistant professor of chemical engineering and biomedical engineering at Penn State, was part of a team that developed a new surface treatment to stop microbes from adhering to catheters. Credit: Kate Myers/Penn State. All Rights Reserved.
Scientists devise method to help prevent hospital infections
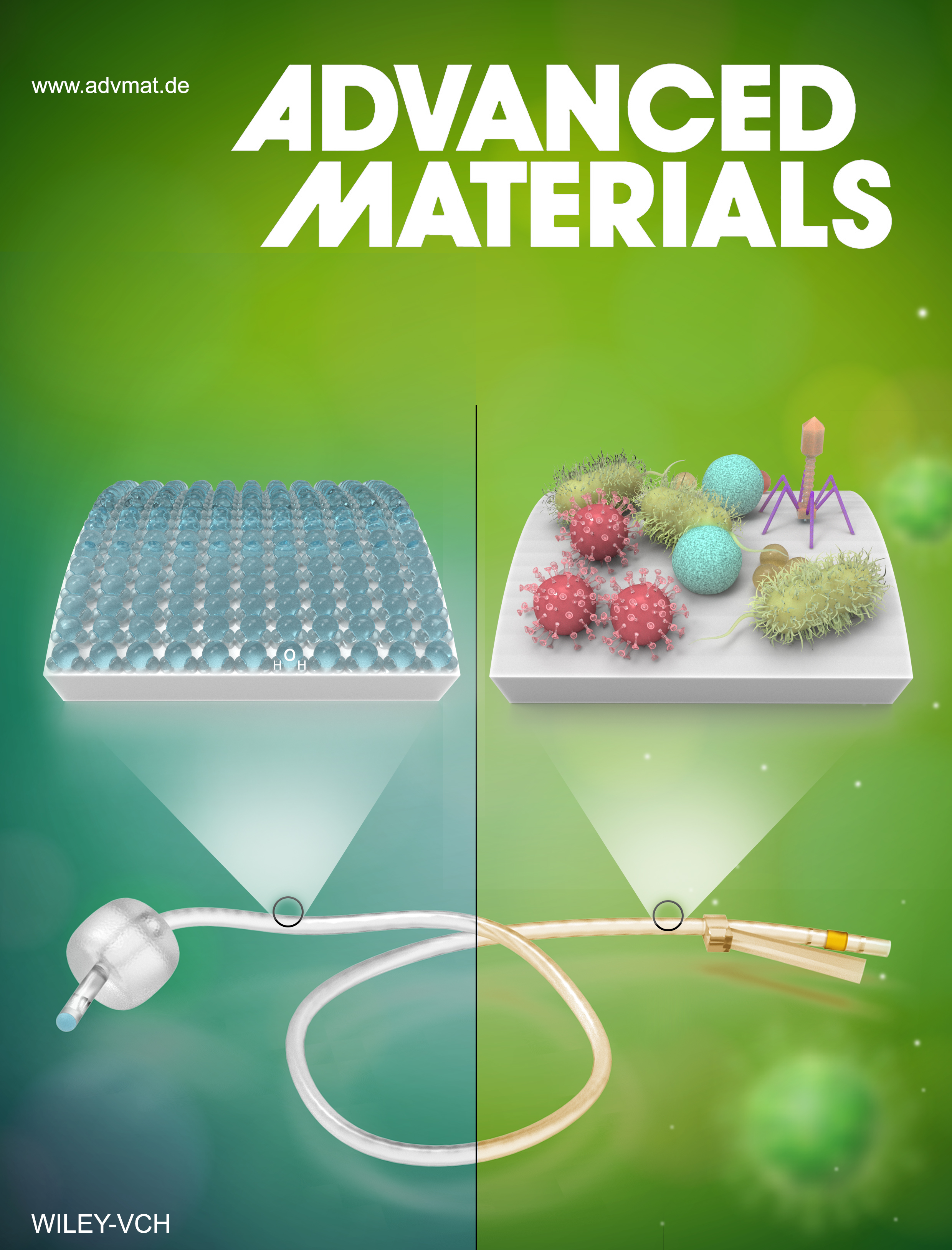
Novel surface treatment stops microbes from adhering to catheters
June 6, 2022
UNIVERSITY PARK, Pa. — On any given day, one in 31 hospital patients is diagnosed with an infection that developed as a result of care during their hospital stay, according to the Centers for Disease Control and Prevention. Medical devices such as catheters, stents, heart valves and pacemakers, whose surfaces can become covered with harmful bacterial films, account for about a quarter of such infections. To help prevent such infections, a research team led by Penn State and the University of California, Los Angeles, developed a novel surface treatment for these devices.
The team’s findings were published in the May 19 issue of Advanced Materials.
The new approach, tested in both laboratory and clinical settings, involves depositing a thin layer of what is known as zwitterionic material on the surface of a device and using ultraviolet light irradiation to permanently bind that layer to the underlying substrate. The resulting barrier prevents bacteria and other potentially harmful organic materials from adhering to the surface and causing infection.
In the laboratory, researchers applied the surface treatment to several commonly used medical device materials, then tested the modified materials’ resistance to various types of bacteria, fungi and proteins. They found that the treatment reduced biofilm growth by more than 80% — and in some cases up 93%, depending on the microbial strain.
“We have conducted extensive biological assessments to demonstrate the efficacy of a zwitterionic surface treatment in preventing the adhesion of proteins, bacteria and cells to medical device surfaces,” said co-corresponding author Amir Sheikhi, assistant professor of chemical engineering and biomedical engineering at Penn State. “We have also assessed the cytotoxicity and hemolysis of this novel treatment and observed no significant toxicity or hemolytic activity.”
Silicone catheters with the zwitterionic surface treatment were tested in 16 long-term urinary catheter users who switched to silicone catheters with the new zwitterionic surface treatment. Ten of the patients described their urinary tract condition using the surface-treated catheter as “much better” or “very much better,” and 13 chose to continue using the new catheter over conventional latex and silicone options after the study period ended.
“Such catheter-related urinary tract problems are illustrative of the issues plaguing other medical devices, which, once inserted or implanted, can become breeding grounds for bacteria and harmful biofilm growth,” said co-corresponding author Richard Kaner, UCLA’s Dr. Myung Ki Hong Professor of Materials Innovation, distinguished professor of chemistry and biochemistry, and of materials science and engineering. Kaner is also a member of the California NanoSystems Institute at UCLA and affiliated with SILQ Technologies Corp.
The pathogenic cells pumped out by these highly resilient biofilms then cause recurring infections in the body, resulting in routine use of antibiotics.
“Antibiotics are typically used to treat complications originated from infections,” Sheikhi said. “However, microbes have been evolving resistance to antibiotics over time to spawn ‘superbugs.’ We need to maintain global antimicrobial stewardship by seeking ways to prevent infection without the overuse of antibiotics. Preventing the adhesion of bacteria to medical devices will reduce infections, which will in turn reduce the necessity of using antibiotics, thus reducing the chance of antimicrobial resistance emergence.”
Sheikhi said that while there are implications for non-medical applications of this surface treatment technique, such as improving lithium-ion battery performance, the aspect that he is most excited about is the immediate improvement in patient care.
“Our goal is to reduce infection and complications from implanted medical devices that are known to cause morbidity, mortality and a large financial burden on health care systems,” he said. “The surface-treated medical devices, particularly a Foley catheter, were tested in human clinical trials with significant success. The comfort of patients is the most exciting part of this research.”
The National Institutes of Health, the National Science Foundation, the Canadian Institutes of Health Research, SILQ Technologies Corp. and the UCLA Sustainability Grand Challenge funded this research.
Other authors on this paper include Brian McVerry and Ethan Rao of SILQ Technologies Corp. and UCLA; Na He, Paige Curson, Mackenzie Anderson, Alexandra Polasko, Shaily Mahendra, Dino Di Carlo, Reihaneh Haghniaz, Praveen Bandaru, Chueh-Wu Yu, Dayong Chen and Pia Ramos of UCLA; Arey Sayegh and Evgeniy Kreydin of the University of Southern California and Rancho Los Amigos National Rehabilitation Center; Joel Hayashi now of the Novartis Research Foundation but of UCLA at the time of research; and Ali Khademhosseini, now of Teraski Institute but of UCLA at the time of research.
The original press release can be viewed at UCLA’s newsroom.