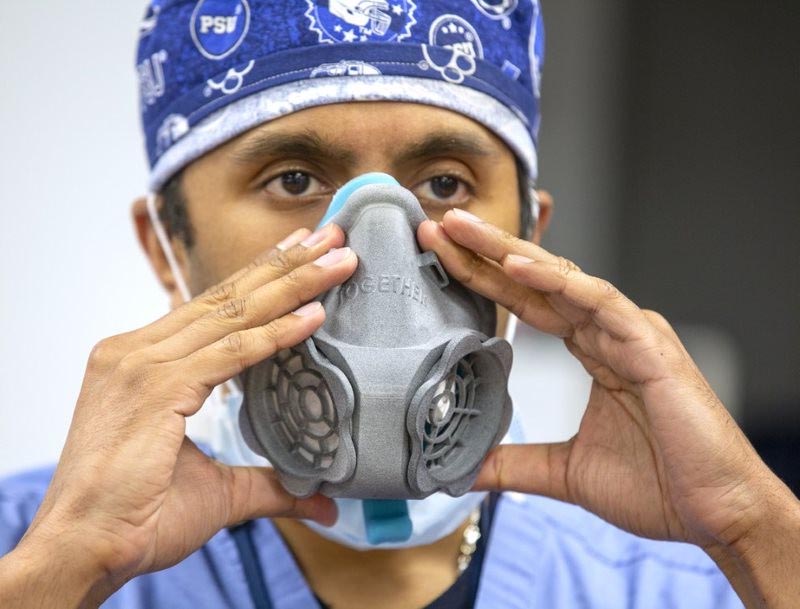
Dr. Neerav Goyal, a physician at the Milton S. Hershey Medical Center, tests out a 3D-printed filtration mask prototype. IMAGE: JASON PLOTKIN
COVID-19 response at Penn State propelled by interdisciplinary connections
The Manufacturing and Sterilization for COVID-19 Initiative (MASC) develops personal protective equipment, testing supplies for Pennsylvania hospitals with FDA compliance
4/22/2020
By Erin Cassidy Hendrick
UNIVERSITY PARK, Pa. — In response to the ongoing pandemic, the Manufacturing and Sterilization for COVID-19 (MASC) Initiative was launched at Penn State in March. With more than 350 researchers contributing, the initiative is focused on designing and delivering rapidly scalable solutions and generating tangible impact, particularly within the commonwealth of Pennsylvania.
MASC reached a milestone on April 7, when a group of physicians gathered at Penn State Health Milton S. Hershey Medical Center to meet virtually with faculty members. They were presented with several 3D-printed protective mask designs to test and provided direct feedback on fit, size and comfort.
“The energy at the prototyping sessions was electric,” said Dr. Neerav Goyal, a physician at the Milton S. Hershey Medical Center, who attended the event. “It is clear that the University researchers are very interested in translating their research expertise into real-world solutions for the current public health crisis.”
MASC was well poised for early impacts, thanks to the University’s broad network. In early March, MASC partnered with the College of Medicine and their Center for Medical Innovation’s Accelerating Innovation in a Crisis program that committed substantial resources to solving clinical needs at Penn State Health.
“It has been exciting to be speaking with engineers who are able to think outside of clinical constraints and current practices,” Goyal added.
Tim Simpson, professor of mechanical engineering and industrial and manufacturing engineering, who is spearheading MASC, explained a distinguishing feature of their work has been careful consideration and monitoring of U.S. Food and Drug Administration (FDA) regulations.
Simpson said, “What we knew from the start is that Penn State can mobilize the rapid innovations needed to design these supplies. Then, we can build out the supply chain with our industry partners near each hospital to physically produce the items and ensure FDA compliance.”
While the initial prototypes were 3D printed, in order to scale manufacturing to meet the current demand, several traditional manufacturing companies, with existing certifications and FDA compliance, were recruited to create the mask’s shell.
However, the devices still required a filtration material within the shell.
MASC contributors, including Sue Purdum from the Center for Supply Chain Research within the Smeal College of Business, sourced a large supply of a non-woven textile that met the specifications for filtering particles in the air. While the material has not previously been used for face masks, Simpson said that after careful testing by experts in the Materials Research Institute, the material was found to provide the necessary protection.
With all the components in place, Simpson hopes the masks will enter production, in order to supply facilities such as Hershey Medical Center and Penn State Health St. Joseph Medical Center in Reading.
Additional MASC teams have also celebrated milestones.
Safely recycling N95 masks
By using a combination of vaporized hydrogen peroxide and plasma sterilization, College of Medicine researchers have gained the ability to decontaminate and recycle existing N95 masks for re-use at Hershey Medical Center.
Creation of intubation booth
Spearheaded by a team formed in the Applied Research Laboratory (ARL), an intubation booth has been designed, prototyped and tested for doctors to utilize while intubating infected patients. By providing a translucent structure around the patient’s upper body, physicians predict the barrier will limit the spread of contaminants during the intubation process. Researchers hope to deliver 10 finalized booths by mid-April.
3D printing test swabs
A critical need to battle the virus’ spread includes testing swabs, essentially long flexible Q-tips that are inserted deep into the nasal cavity to collect a sample. Without them, doctors are limited in their ability to properly diagnose the virus.
Researchers are close to a final prototype, utilizing 3D printing. Once again echoing MASC’s commitment to FDA compliance, their final design will be handed off to an approved facility for manufacturing, which has the ability to produce thousands per day.
Delivery and design of powered air purifying respirators (PAPR)
A MASC team also enabled the delivery of PAPR, a type of personal protective equipment that purifies and recycles air through a hood and mask to provide additional protection from airborne contaminants. This week, five traditionally manufactured PAPRs that had previously been used in Simpson’s lab for prior research, were delivered to St. Joseph Medical Center.
Taking it one step further, once again to address supply chain issues, specialists from ARL on the MASC team helped design and prototype a PAPR that can be built with supplies found easily at hardware stores. Their prototypes are currently being testing at the facility.
Seeing these successes emerge in a few short weeks inspires Simpson and the more than 300 Penn State stakeholders working within MASC to continue.
“We now span 21 commonwealth campuses and multiple colleges, all with the hope of having direct and immediate impact on our communities,” he said. “We are leveraging our Penn State connections across the entire state.”
To support Penn State’s response to the novel coronavirus pandemic, visit c-fund.us/rhc.



